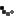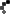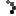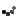As allergy season approaches, the FDA is urging consumers to double-check their medicine cabinets. The recall of SinuCleanse Soft Tip Squeeze Bottle Nasal Wash System, initially announced in February, has now been upgraded to a Class 1 recall—the most serious classification, indicating a potential risk of serious injury or death.
The recall was voluntarily issued by Ascent Consumer Products Inc. after one lot of the nasal wash tested positive for Staphylococcus aureus (staph), a common bacterium that can cause life-threatening infections if it enters the bloodstream, especially through nasal irrigation.
The affected lot, #024122661A1, has a best-by date of December 31, 2027, and was distributed nationwide in January 2025, both in stores and online. If you have this product, do not use it. Instead, dispose of it safely or return it to the retailer.
According to the FDA, staph infections from contaminated nasal irrigation can lead to dangerous complications, including blood infections, meningitis, endocarditis (heart lining infection), bone infections, and even vision loss. Those with irritated or injured nasal tissue are particularly at risk.
If you’ve already used the recalled product and are experiencing any unusual symptoms, contact your healthcare provider right away.
The FDA explains that while this classification update may seem delayed, it follows standard procedures for public safety and transparency. The agency begins alerting the public as soon as voluntary recalls are issued, with final classifications coming after thorough assessment.
Consumers with questions can reach Ascent Consumer Products at cs@ascentconsumerproducts.com, Monday through Friday, 9 a.m. to 5 p.m. EST.
Your health matters—staying informed is the first step toward staying safe.