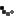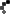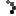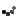Roughly 14,250 packs of Safetussin Max Strength Multi-Symptom Cough, Cold and Flu have been recalled due to packaging that fails to meet child-resistant standards, posing a serious risk of accidental poisoning. Kramer Laboratories, the manufacturer, announced the voluntary recall after the Consumer Product Safety Commission (CPSC) flagged that children could easily push tablets through the foil backing.
The medicine contains acetaminophen, a common but potentially dangerous drug for children if consumed in large amounts. Under the Poison Prevention Packaging Act, products with acetaminophen must be sold in child-resistant containers.
The recall affects 24-count blister packs sold between July 2024 and March 2025 at independent pharmacies and regional grocery stores nationwide. Packaging reads: “Safetussin,” “Multi-Symptom,” “Cough, Cold & Flu,” and notes it is “Safe for adults with High Blood Pressure, Diabetes.” Retail price: around $11.
Consumers are urged to keep the product out of reach of children and contact Kramer Laboratories for disposal and a refund. While the medicine itself is not defective, both the packaging and product should be discarded. For more information, visit safetussin.com/recall or call 800-824-4894.
This isn’t the first time cold medications have been pulled due to child safety hazards. In 2023, Walgreens and Rite Aid recalled store-brand children’s cough syrups after dosing cups lacked proper markings. In another case, more than 83,000 bottles of Theraflu products were recalled in 2022 for similar packaging violations.
This recall is a strong reminder to double-check your medicine cabinet—especially if you have young children at home.